Next we consider nonstationary signals which consist of segments with
identical standard deviation (![]() ) but different correlations. We
obtain such
signals using the following procedure: (1) we generate two stationary signals
) but different correlations. We
obtain such
signals using the following procedure: (1) we generate two stationary signals
![]() and
and ![]() (see Sec. 2) of identical length
(see Sec. 2) of identical length
![]() and with different correlations, characterized by scaling exponents
and with different correlations, characterized by scaling exponents
![]() and
and ![]() ; (2) we divide the signals
; (2) we divide the signals ![]() and
and
![]() into non-overlapping segments of size
into non-overlapping segments of size ![]() ; (3) we randomly replace
a fraction
; (3) we randomly replace
a fraction ![]() of the segments in signal
of the segments in signal ![]() with the corresponding
segments of
with the corresponding
segments of ![]() . In Fig. 5(a), we show an example of such a
complex nonstationary signal with different local correlations. In this
Section, we study the behavior of the r.m.s. fluctuation function
. In Fig. 5(a), we show an example of such a
complex nonstationary signal with different local correlations. In this
Section, we study the behavior of the r.m.s. fluctuation function
![]() . We also investigate
. We also investigate ![]() separately for each
component of the nonstationary signal (which consists only of the segments
with identical local correlations) and suggest an approach, based on the
DFA results, to recognize such complex structures in real data.
separately for each
component of the nonstationary signal (which consists only of the segments
with identical local correlations) and suggest an approach, based on the
DFA results, to recognize such complex structures in real data.
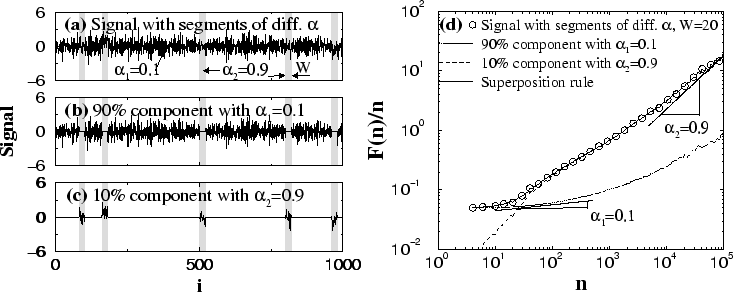 |
In Fig. 5(d), we present the DFA result on such a nonstationary
signal, composed of segments with two different types of local correlations
characterized by exponents
![]() and
and
![]() . We find
that at small scales, the slope of
. We find
that at small scales, the slope of ![]() is close to
is close to ![]() and at
large scales, the slope approaches
and at
large scales, the slope approaches ![]() with a bump in the
intermediate
scale regime. This is not surprising, since
with a bump in the
intermediate
scale regime. This is not surprising, since
![]() and thus
and thus
![]() is bound to have a small slope (
is bound to have a small slope (![]() ) at small scales and
a large slope (
) at small scales and
a large slope (![]() ) at large scales. However, it is surprising that
although 90% of the signal consists of segments with scaling exponent
) at large scales. However, it is surprising that
although 90% of the signal consists of segments with scaling exponent
![]() ,
, ![]() deviates at small scales (
deviates at small scales (![]() ) from the
behavior expected for an anti-correlated signal
) from the
behavior expected for an anti-correlated signal ![]() with exponent
with exponent
![]() [see, e.g.,
the solid line in Fig. 2(b)]. This suggests that the behavior of
[see, e.g.,
the solid line in Fig. 2(b)]. This suggests that the behavior of
![]() for a nonstationary signal comprised of mixed segments with different
correlations is dominated by the segments exhibiting higher positive
correlations even in the case when their relative fraction in the signal is
small. This observation is pertinent to real data such
as: (i) heart rate recordings during sleep where different segments
corresponding to different sleep stages exhibit different types of
correlations[33]; (ii) DNA sequences including coding and
non-coding regions characterized by different correlations[5,8,16]
and (iii) brain wave signals during different sleep stages[62].
for a nonstationary signal comprised of mixed segments with different
correlations is dominated by the segments exhibiting higher positive
correlations even in the case when their relative fraction in the signal is
small. This observation is pertinent to real data such
as: (i) heart rate recordings during sleep where different segments
corresponding to different sleep stages exhibit different types of
correlations[33]; (ii) DNA sequences including coding and
non-coding regions characterized by different correlations[5,8,16]
and (iii) brain wave signals during different sleep stages[62].
To better understand the complex behavior of ![]() for such nonstationary
signals, we study their components separately. Each component is composed
only of those segments in the original signal which are characterized by
identical correlations,
while the segments with different correlations are substituted with zeros [see
Figs. 5(b) and (c)]. Since the two components of the nonstationary
signal in Fig. 5(a) are independent, based on the superposition rule
[Eq. (5)], we expect that the r.m.s. fluctuation function
for such nonstationary
signals, we study their components separately. Each component is composed
only of those segments in the original signal which are characterized by
identical correlations,
while the segments with different correlations are substituted with zeros [see
Figs. 5(b) and (c)]. Since the two components of the nonstationary
signal in Fig. 5(a) are independent, based on the superposition rule
[Eq. (5)], we expect that the r.m.s. fluctuation function ![]() will behave as
will behave as
![]() , where
, where ![]() and
and
![]() are the r.m.s. fluctuation functions of the components in
Fig. 5(b) and Fig. 5(c), respectively. We find a remarkable
agreement between the superposition rule prediction and the result of the DFA
method obtained directly from the mixed signal [Fig 5(d)]. This
finding helps us understand the relation between the scaling behavior of
the mixed nonstationary signal and its components.
are the r.m.s. fluctuation functions of the components in
Fig. 5(b) and Fig. 5(c), respectively. We find a remarkable
agreement between the superposition rule prediction and the result of the DFA
method obtained directly from the mixed signal [Fig 5(d)]. This
finding helps us understand the relation between the scaling behavior of
the mixed nonstationary signal and its components.
Information on the effect
of such parameters as the scaling exponents ![]() and
and ![]() ,
the size of the segments
,
the size of the segments ![]() and their relative fraction
and their relative fraction ![]() on the scaling
behavior of the components provides insight into the scaling
behavior of the
original mixed signal. When the original signal comes from real data,
its composition is a priori unknown. A first step is to ``guess'' the
type of correlations (exponents
on the scaling
behavior of the components provides insight into the scaling
behavior of the
original mixed signal. When the original signal comes from real data,
its composition is a priori unknown. A first step is to ``guess'' the
type of correlations (exponents ![]() and
and ![]() ) present in the
signal, based on the scaling behavior of
) present in the
signal, based on the scaling behavior of ![]() at small and large scales
[Fig 5(d)]. A second step is to determine the parameters
at small and large scales
[Fig 5(d)]. A second step is to determine the parameters ![]() and
and ![]() for each component by matching the scaling result from the superposition rule
with the original signal. Hence in the following subsections, we focus on the
scaling properties of the components and how they change with
for each component by matching the scaling result from the superposition rule
with the original signal. Hence in the following subsections, we focus on the
scaling properties of the components and how they change with ![]() ,
, ![]() and
and ![]() .
.