These findings indicate that fractal gait dynamics depend on central nervous system function. Therefore, we hypothesized that just as aging and cardiovascular disease may alter the fractal nature of the heartbeat, so too changes in central nervous system function might alter the fractal gait pattern. To test this hypothesis, we have begun to systematically study the effects of advanced age and neurodegenerative disorders on fractal gait rhythm [36].
Effects of Aging
We compared the gait of a group of very healthy elderly adults
(ages 76 ![]() 3 yrs) to healthy young adults (ages 25
3 yrs) to healthy young adults (ages 25 ![]() 2
yrs). Interestingly, both groups had identical mean stride intervals
(elderly: 1.05 sec; young: 1.05 sec), and required almost identical
amounts of time to perform a standardized functional test of gait and
balance. The magnitude of stride-to-stride variability (i.e., stride
interval coefficient of variation) was also very similar in the two
groups (elderly: 2.0%; young: 1.9 %). Fig. 11 (
left) compares the stride interval time series for a young and an
elderly subject. Visual inspection suggests a possible subtle
difference in the dynamics of the two time series (the data from the
young subject appearing more ``patchy''). Fluctuation analysis reveals
a marked distinction in how the fluctuations change with time scale
for these subjects.
The stride interval fluctuations are more random (less correlated) for the elderly subject than for the young subject, a difference
not detectable by comparing the first and second moments.
2
yrs). Interestingly, both groups had identical mean stride intervals
(elderly: 1.05 sec; young: 1.05 sec), and required almost identical
amounts of time to perform a standardized functional test of gait and
balance. The magnitude of stride-to-stride variability (i.e., stride
interval coefficient of variation) was also very similar in the two
groups (elderly: 2.0%; young: 1.9 %). Fig. 11 (
left) compares the stride interval time series for a young and an
elderly subject. Visual inspection suggests a possible subtle
difference in the dynamics of the two time series (the data from the
young subject appearing more ``patchy''). Fluctuation analysis reveals
a marked distinction in how the fluctuations change with time scale
for these subjects.
The stride interval fluctuations are more random (less correlated) for the elderly subject than for the young subject, a difference
not detectable by comparing the first and second moments.
Similar results were obtained for other subjects in these groups, indicating a subtle, previously undetected alteration in the fractal scaling of gait with healthy aging. Even among healthy elderly adults who have otherwise normal measures of gait and lower extremity function, the fractal scaling pattern is significantly altered when compared with young adults.
From a practical clinical perspective, the breakdown of long-range correlations of gait with aging is of interest for a number of reasons. An exciting prospect is that quantitative assessment of fractal properties of locomotion may provide a simple, inexpensive way to obtain important information about gait instability among the elderly. Falls are a major cause of disability and death in this age group [37]. The ability to identify individuals at greatest risk, as well as to assess interventions designed to restore gait stability (e.g., exercise, footwear), could have major public health implications. From a more basic physiologic viewpoint, realistic models of gait dynamics must account not only for the unexpected long-range correlations in stride interval in health, but also for their breakdown with aging and disease [15].
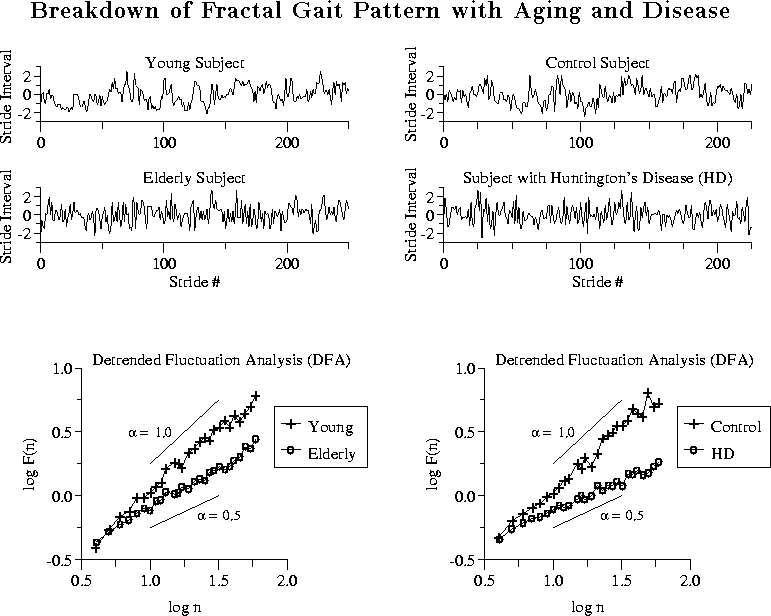
Figure: Left: Example of the effects of aging.
Stride interval time series are
shown (above) and DFA (below) for a 71-year-old elderly
subject and a 23-year-old young adult. For illustrative purposes,
each time series is normalized by subtracting its mean and dividing by
its standard deviation. This normalization process highlights any
temporal ``structure'' in the time series, but does not affect the
fluctuation analysis. Therefore, in this figure, stride interval is
unitless. For the elderly subject, DFA indicates a
more random and less correlated time series. Indeed, ![]() is 0.56
(
is 0.56
(![]() white noise) for the elderly subject and 1.04 (
white noise) for the elderly subject and 1.04 (![]() noise) for the young adult.
Right:
Example of the effects of Huntington's disease (HD).
For the subject with Huntington's disease (age: 41 years old), as compared with a healthy control, the stride interval fluctuations, F(n), increase
more slowly with time scale, n. This indicates a more random and less
correlated time series. Indeed,
noise) for the young adult.
Right:
Example of the effects of Huntington's disease (HD).
For the subject with Huntington's disease (age: 41 years old), as compared with a healthy control, the stride interval fluctuations, F(n), increase
more slowly with time scale, n. This indicates a more random and less
correlated time series. Indeed, ![]() is 0.40 for this subject with
Huntington's disease and 0.92 for this healthy control subject.
Adapted from [36].
is 0.40 for this subject with
Huntington's disease and 0.92 for this healthy control subject.
Adapted from [36].
Effects of Neurodegenerative Disease
We further hypothesized that impaired central nervous system control
might also alter the fractal property of gait. To test this
hypothesis, we have compared the stride interval time series of subjects with Huntington's disease and
Parkinson's disease, two major neurodegenerative disorders of the
basal ganglia (a part of the brain responsible for regulating motor
control), with data from healthy controls. The time series and
fluctuation analysis for a subject with Huntington's disease and a
control subject are shown in Fig. 11 (right panel). For
the subject with Huntington's disease, stride interval fluctuations,
F(n), increase slowly with time scale, n, compared to a healthy
control. This finding indicates increased randomness and reduced
stride interval correlations as compared with the control subject. In
general, compared to healthy control subjects, fractal scaling was
reduced in the subjects with Parkinson's disease and reduced further
in subjects with Huntington's disease. Interestingly, while ![]() was lowest in subjects with Huntington's and intermediate in subjects
with Parkinson's disease, subjects with Parkinson's disease walked
more slowly compared to subjects with Huntington's disease, further
confirming that the mechanisms responsible for the generation of gait
speed are apparently independent of those regulating fractal scaling
(Fig. 10A).
was lowest in subjects with Huntington's and intermediate in subjects
with Parkinson's disease, subjects with Parkinson's disease walked
more slowly compared to subjects with Huntington's disease, further
confirming that the mechanisms responsible for the generation of gait
speed are apparently independent of those regulating fractal scaling
(Fig. 10A).
Among the subjects with Huntington's disease, the fractal scaling
index ![]() was inversely correlated with disease severity (see
Figure 12). Moreover,
was inversely correlated with disease severity (see
Figure 12). Moreover, ![]() was significantly lower in
subjects with the most advanced stages of Huntington's disease as
compared with subjects in the early stages of the
disease,
indicative of more random stride interval fluctuations.
Interestingly, in a few subjects with the most severe
impairment,
was significantly lower in
subjects with the most advanced stages of Huntington's disease as
compared with subjects in the early stages of the
disease,
indicative of more random stride interval fluctuations.
Interestingly, in a few subjects with the most severe
impairment, ![]() was less than 0.5, suggesting the presence of a
qualitatively different type of dynamical behavior (namely,
anti-correlations) in the gait rhythm.
was less than 0.5, suggesting the presence of a
qualitatively different type of dynamical behavior (namely,
anti-correlations) in the gait rhythm.
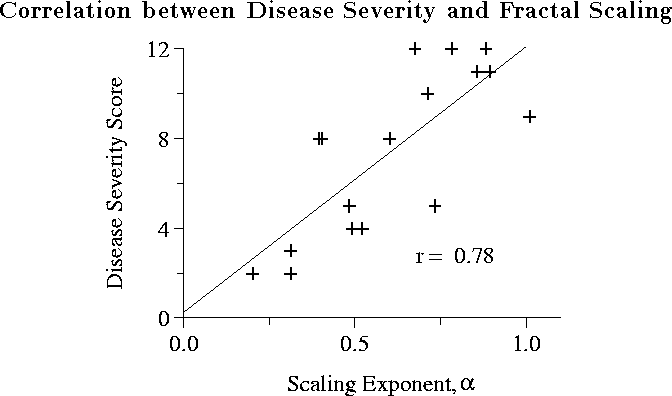
Figure: Among subjects with
Huntington's disease, disease severity score (0=most impairment; 13=no
impairment), measured using an index that correlates with positron
emission tomography (PET) scan indices of caudate metabolism
[38], is strongly (p < .0005) associated with
fractal scaling of gait. Adapted from [36].
These results indicate that with both Parkinson's and Huntington's disease, there is a breakdown of the normal fractal, long-range correlations in the stride interval, especially apparent in subjects with advanced Huntington's disease. Step-to-step fluctuations are more random (i.e., more like white noise), suggesting that the fractal property of gait is modulated in part by central nervous system (i.e., basal ganglia) function. Although fractal scaling is altered both with aging and certain diseases, the magnitude of these changes varies in different conditions, and other measures of gait dynamics may also distinguish among different disease states and aging [39], adding specificity to these new dynamical measures (compare with Fig. 6).